Progress towards eliminating malaria has stalled. New solutions are needed to prevent, detect and treat one of the world’s biggest health threats.
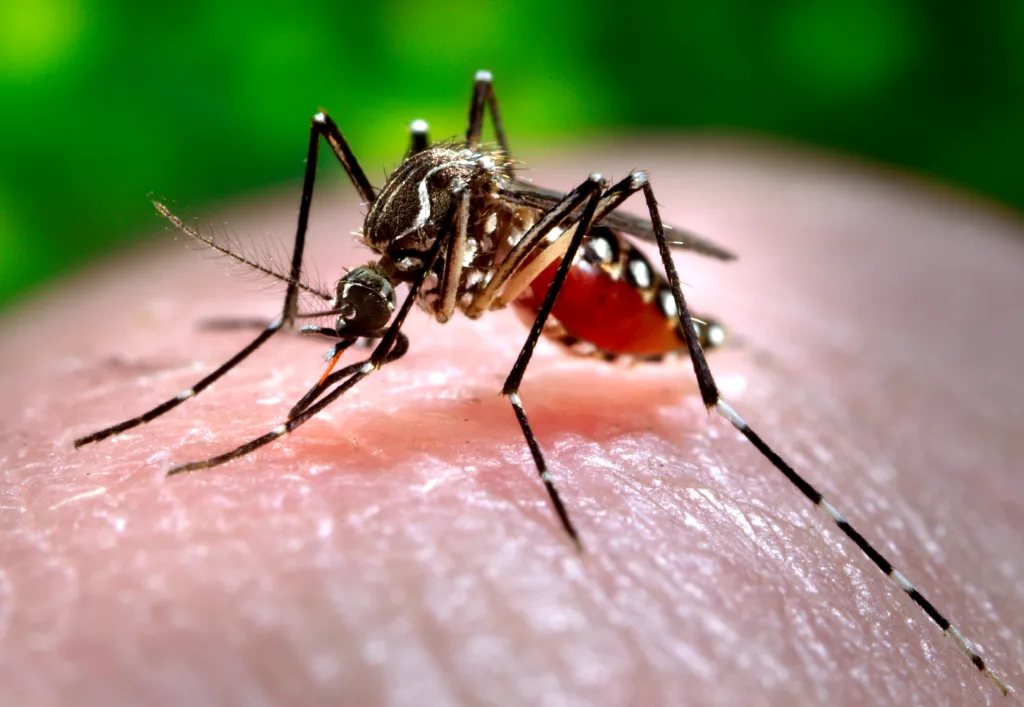
 Insecticide-treated bed nets have helped prevent malaria infections in the past but are becoming less effective. : CDC: BK Kapella, MD (CDR; USPHS) Public domain
Insecticide-treated bed nets have helped prevent malaria infections in the past but are becoming less effective. : CDC: BK Kapella, MD (CDR; USPHS) Public domain
Progress towards eliminating malaria has stalled. New solutions are needed to prevent, detect and treat one of the world’s biggest health threats.
Every minute, more than 400 people develop malaria, and around every minute, a person dies because of it.
Most likely, it’s a child under five years old in sub-Saharan Africa. Children in Africa account for around 70 percent of malaria deaths, although there are substantial burdens of the disease in the Asia-Pacific and parts of the Americas.
Yet despite the risk malaria poses to 40 percent of the world’s population and ambitious targets being set to eliminate it, progress in reducing the global burden of the disease has stalled since around 2015.
Alarmingly, the global malaria burden increased in 2020, due in part to the impacts of the COVID-19 pandemic. The 2023 World Malaria Report estimated there were 249 million cases of malaria in 2022.
New strategies and tools are needed to accelerate progress towards achieving malaria elimination — through better prevention, treatment, diagnosis and surveillance of the disease.
Malaria is not transmitted from person to person, but through the bite of infected Anopheles mosquitoes carrying a Plasmodium parasite (P. falciparum or P. vivax). This presents specific challenges for achieving elimination that differ from many other infections.
Reducing malaria requires strategies that target mosquitoes as well as people, and isolation of infectious cases and social distancing measures used in other infectious diseases are not effective strategies.
A simple and moderately effective way to prevent malaria infection is to sleep under a bed net to avoid mosquito bites.
Bed nets treated with insecticides are even more effective in repelling mosquitoes. Mosquitos will rest indoors after biting for a blood meal, and so they can be killed by coating indoor surfaces with insecticides. Thus, indoor residual insecticide spraying can prevent onward Plasmodium transmission to others.
For more than 20 years, the widespread use of insecticide-treated bed nets and indoor spraying has been essential to achieving progress in reducing the malaria burden.
However, changes in mosquito behaviours and increasing resistance of mosquitoes to insecticides and parasites to antimalarial drugs have reduced the effectiveness of these methods. This has been compounded by incomplete coverage of preventive interventions in high-risk populations.
In the current scenario, a vaccine that is highly protective and could prevent transmission of malaria would be transformative.
The World Health Organization’s recent recommendation of widespread implementation of the first malaria vaccines (RTS,S /AS01 and R21/Matrix-M) represents a major advance.
However, challenges remain. The vaccines are currently recommended for use only in young children and have a relatively short duration of protection, which means boosters are required after 12 to 18 months.
Their efficacy is also affected by the genetic diversity of malaria parasites circulating in populations, so the vaccine may not prevent malaria caused by some strains.
These vaccines are only effective against P. falciparum, which causes the bulk of malaria in Africa, and not P. vivax malaria, which is widely present across the Asia-Pacific region and parts of Africa and the Americas.
Ongoing research is aimed at improving how long the immune system can protect against infection and designing a vaccine that protects against all strains across various geographical locations.
The widespread emergence of Plasmodium parasites, especially P. falciparum, resistant to antimalarial drugs, presents a considerable risk of increasing malaria morbidity and mortality.
Additionally, P. vivax establishes a latent liver infection, which can reactivate weeks or even years after the initial infection, causing relapses of malaria.
Diagnosing and treating these dormant infections remains challenging, another constraint on the progress of malaria control and elimination programmes.
The development of new drugs to combat resistance or the optimisation of combinations of the currently available drugs are different paths to ensure effective antimalarial therapy and patients’ well-being and survival.
In parallel, reliable diagnostic tools can serve various purposes towards malaria control and elimination: early diagnosis and treatment prevents clinical complications, accurate diagnosis guides an appropriate selection of medications, and accurate diagnostic tests aid in monitoring local or seasonal parasite transmission.
While malaria treatment and prevention have been the mainstay for malaria control, to achieve malaria elimination, all malaria infections must be effectively prevented or detected and treated.
Many infections are not detected by routine surveillance or established diagnostic tests, and many infections are asymptomatic and go undiagnosed. As a result, transmission of malaria in populations continues.
The epidemiology of malaria in the Asia-Pacific region, for example, is highly variable and diverse and further complicated by high rates of drug resistance, large reservoirs of undetected infections and diversity in the mosquito species that transmit malaria.
New, highly sensitive and precise markers of malaria infection combined with new tools to define malaria transmission would enable more efficient targeting of malaria by preventive interventions and treatment and the best use of limited resources.
Professor James Beeson is a medical researcher and public health physician. He leads the Burnet Institute’s Malaria Immunity and Vaccines Research Group. Professor Beeson has worked in the field of global health for over 20 years with a focus on infectious diseases and maternal and child health.
Professor Beeson receives research funding from the National Health and Medical Research Council (NHMRC).
Dr Amaya Ortega Pajares is a biomedical scientist specialising in immunology and tropical diseases, and manager of the Cellular Responses to Disease and Vaccination lab at Burnet Institute.
Originally published under Creative Commons by 360info™.