Engineered cells and bacteria offer hope for diabetes, arthritis
Therapy combining a type of engineered blood cells and bacteria that modify the gut microbiome offers promise for managing autoimmune diseases.
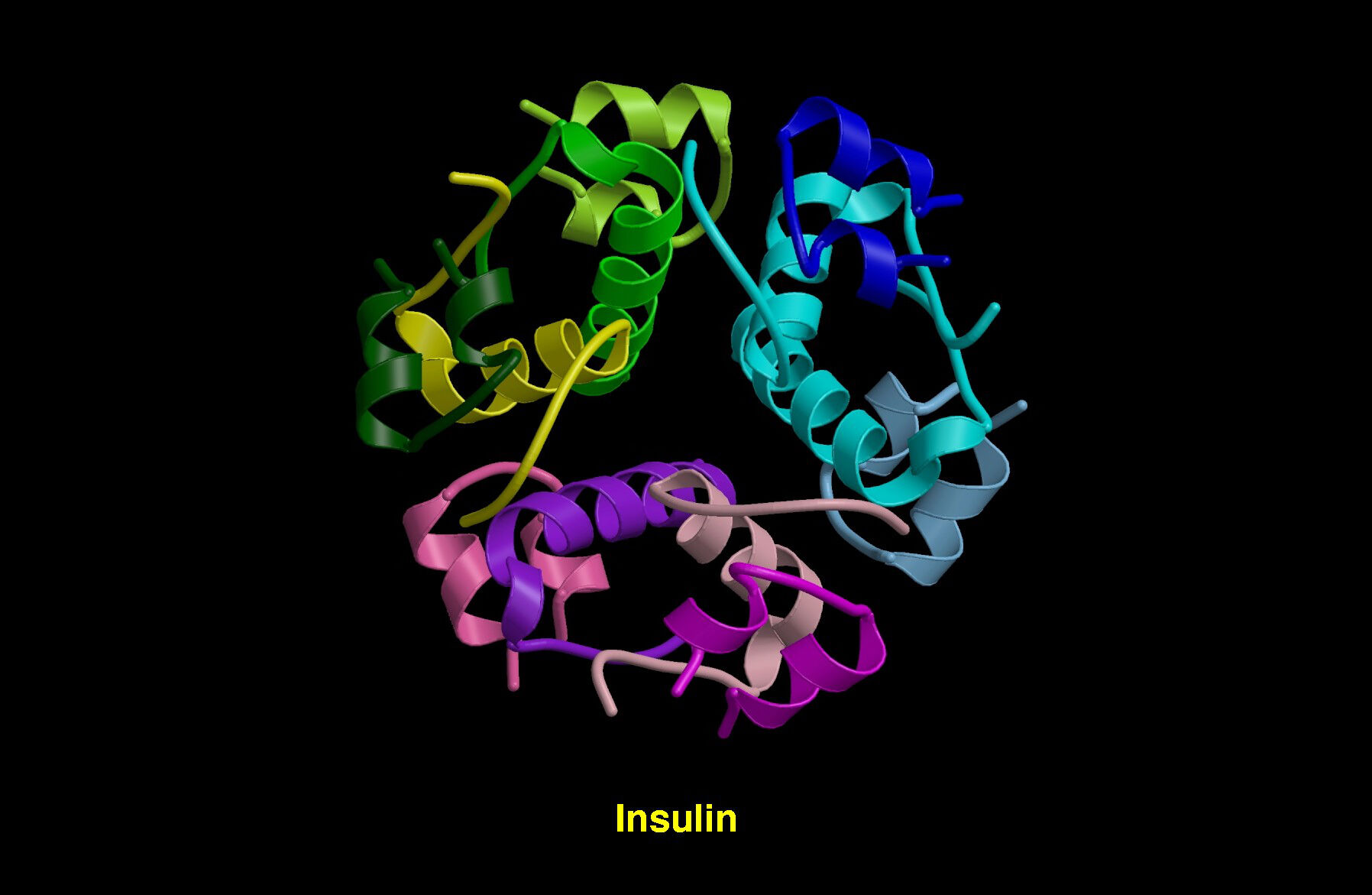 Molecular model of the insulin molecule. Insulin molecules consist of two peptide chains (A and B) linked by bonds : T.Blundell & N Campillo. Source: Wellcome Collection. CC0 1.0 Universal
Molecular model of the insulin molecule. Insulin molecules consist of two peptide chains (A and B) linked by bonds : T.Blundell & N Campillo. Source: Wellcome Collection. CC0 1.0 Universal
Therapy combining a type of engineered blood cells and bacteria that modify the gut microbiome offers promise for managing autoimmune diseases.
Blame it on lifestyles, genetic predispositions, and changes in our environment: autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, lupus and multiple sclerosis are emerging as a significant public health challenge.
Estimates suggest that a staggering 18 per cent of Delhi’s 33 million people are likely to develop some form of autoimmune disease.
Around three to eight percent of people globally are living with an autoimmune disease — although this number is higher in countries such as the UK and seems to be rising in many parts of the world, underlining the widespread nature and threat of these diseases.
There is no cure for any autoimmune disease and therapy options can be limited, so researchers are turning to novel approaches to find new ways to treat them.
Engineered bacteria
One such approach involves engineering a type of white blood cell called regulatory T cells and integrating this with modifications to the gut microbiome using engineered bacteria.
The gut microbiome — the collection of microorganisms that live in the human intestines, including bacteria, viruses, fungi and protozoa — may be a critical factor predisposing individuals to inflammatory disorders.
Our body harbours millions of immune cells that act as sentinels against daily challenges such as microbes and foreign substances and protect us by eliminating these foreign invaders.
Often, these immune cells can go rogue and start attacking the body’s own cells and tissues, mistaking them for threats. This is called an autoimmune disease.
Treating autoimmune diseases is extremely challenging because a fragile balance has to be maintained. Medicines that weaken the immune system too much would leave the body open to attacks by bacteria, viruses and other pathogens.
A new approach
The body has a kind of white blood cells called T cells which protect it against infections and cancers.
There are broadly two types of T cells — one that fights infection and cancer, and another that reduces the cytotoxic or cell-killing activity of the “fighter” T cells once the infection or other triggers subside.
The latter kind of T cells are called regulatory T cells. These are important for controlling autoimmune diseases such as type 1 diabetes by protecting the organs such as the pancreas from damage caused by rogue hyperactive cell-killing T cells.
Regulatory T cells are crucial in regulating the body’s immune response. Their ability to suppress the immune system can be a potent tool to treat chronic inflammatory autoimmune disease.
Restoration of these regulatory T cells in autoimmune diseases has proved beneficial in reducing disease severity in preclinical animal models but has seen limited success in humans.
One reason for their lack of efficacy has been poor targeting. Only a small portion of these cells target the organ under attack by the rogue cells.
To aim these cells at the target organs, they can be engineered to recognise specific antigens or signature molecules that are expressed in the organs under disease conditions.
For instance, molecules of insulin B chain 10–23 peptides are found in large quantities in pancreas with type-1 diabetes. Regulatory T cells have been engineered to target this specific antigen, with much greater efficacy than their normal counterparts.
Engineering these antigen-specific regulatory T cells in a lab and then transferring such engineered cells into a patient’s body to treat autoimmune diseases such as diabetes is an exciting possibility.
Tailored treatment
This treatment strategy can be tailored to individual patients.
The regulatory T cells can be engineered to target specific antigens that are unique to a patient and upregulated in various autoimmune diseases.
This therapy can be a safer alternative to traditional therapies such as prescription drugs like anti-inflammatories or immunosuppressive drugs, as unlike those, engineered regulatory T cells do not suppress the whole immune system.
The therapy has shown promising results in the treatment of autoimmune disorders like type 1 diabetes and autoimmune arthritis in early-stage clinical trials. However, it has yet to reach clinics to benefit patients.
Challenges
One of the challenges in successfully using this type of treatment is identifying factors that can enhance the efficacy of these cells in controlling inflammation in autoimmune disease patients.
Identifying the optimal unique antigens or signatures associated with autoimmune diseases can improve our ability to design better-engineered regulatory T cells for therapeutic purposes.
One such enzyme in the human body is called “eukaryotic elongation factor 2 kinase” or eEF2K. This controls T cell activity and initiates cell-killing T cell responses, leading to autoimmune disorders.
Mice deficient in this protein showed an increase in aberrant and cytotoxic T-cell response, which came at the expense of the functionality of regulatory T cells.
eEF2 kinase could be a potential factor to be targeted for improving outcomes in adoptive regulatory T-cell therapy.
Gut microbiome and immunity
Effective therapies against autoimmune diseases to improve disease outcomes would need to tackle multiple factors. One could be the gut microbiota which contains trillions of microbes that play a major role in modulating immune responses.
Adoptive regulatory T-cell therapy in combination with gut microbial modulation could make major inroads into autoimmune immunotherapy.
The hygiene hypothesis indicates that high standards of hygiene limit exposure to beneficial microbes, making people prone to develop a hyper-alert immune system, thus leading to autoimmune diseases.
The interactions between our gut microbiota and immune system are complex. If the gut microbiome is depleted, anti-inflammatory regulatory T cells may be suppressed while cell-killing T cells that attack organs like the pancreas are inducted. This can cause type 1 diabetes.
Bacteria have been used to replenish the balance of the gut microbiota and produce certain beneficial compounds or metabolites. These compounds change immunological tolerance for the better and enhance a pro-inflammatory microenvironment. This helps ameliorate autoimmune diseases like rheumatoid arthritis, as reported in this study.
Here, the metabolically engineered bacterium is used to improve the efficacy of immunotherapy, defeat autoimmunity, and reduce inflammation in a pre-clinical model of autoimmune arthritis. The engineered bacterium augmented the function of regulatory T cells to defeat artificially induced autoimmune rheumatoid arthritis in mice.
Adoptive regulatory T-cell therapy and bacterial therapy to modulate the microbiome are exciting avenues for treating autoimmune diseases. These therapies have a common thread of using the body’s natural regulatory mechanisms to counter autoimmunity.
Although both therapies are still in the early stages of research and development, their potential to provide safer and more effective treatments than conventional immunosuppressive strategies is significant.
Dr Jugal Das is the Ramalingaswami Fellow in the Faculty of Natural Sciences at Shiv Nadar Institution of Eminence, Delhi-NCR.
Dr Aryashree Arunima is a Postdoctoral Research Associate at Texas A&M University’s College of Medicine.
Dr Shrijita Banerjee is an Academic Associate with the Department of Life Sciences at Shiv Nadar Institution of Eminence, Delhi-NCR.
Originally published under Creative Commons by 360info™.